Four Things I Learned from Visiting Argonne National Laboratory
More on:
For seventy years, Argonne has hosted cutting-edge scientific research. The first national laboratory in the United States, Argonne was created in 1946 as an extension of the Manhattan Project to develop nuclear technology. Today, its research spans high-energy physics, supercomputing, and advanced materials, but I paid a visit to Argonne last month for one reason in particular: the Laboratory has established itself as a thriving hub for research on battery energy storage.
To write his 2015 book, The Powerhouse: Inside the Invention of a Battery to Save the World, Quartz reporter Steve Levine spent two years embedded in Argonne’s battery laboratories, documenting a thrilling race to develop the next-generation lithium-ion battery. I was particularly fascinated by the central but confusing role that Argonne found itself. As a national laboratory, its core competency is basic science research and development, at which its scientists excel. But on top of that, the Lab has developed external partnerships with the private sector, for example, by licensing technology to General Motors to build the battery for its Volt electric vehicle (Argonne technology may soon emerge in next year’s 238-mile-range Chevy Bolt). And it found itself under intense pressure to deliver results after the Department of Energy designated it as the U.S. energy innovation hub for energy storage. Arguably, a transformative advance in energy storage is the most important clean energy technology need in the quest to confront climate change and secure energy independence.
But Argonne, along with a swathe of Silicon Valley start-ups, has struggled to commercialize breakthrough battery technologies. Today, it remains unclear whether a credible challenger can unseat the formidable lithium-ion battery, which Asian firms (and more recently, Tesla) are churning out en masse. Still, on my visit I encountered healthy doses of optimism—tempered by experience with research dead-ends—and a trove of lessons for how to commercialize emerging technologies. Here are four of them:
1. Lithium-sulfur batteries are the best bet to succeed lithium-ion
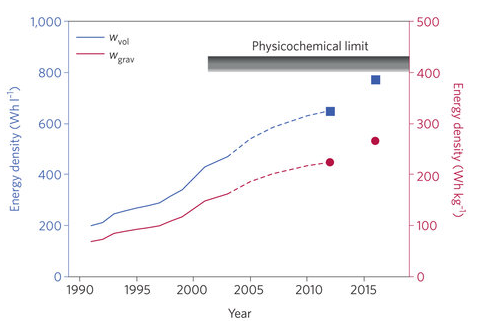
Researchers at Argonne’s Joint Center for Energy Storage Research (JCESR) are racing to deliver an electric-vehicle battery prototype that will pack more energy per pound, at a lower price point, than anything on the market. Lithium-ion batteries currently dominate energy storage, encompassing mobile phones, electric vehicles, and power grids, but their performance is approaching theoretical limits (figure 1). JCESR researchers—after chasing a dead-end in hyped but finicky lithium-air technology—now believe lithium-sulfur will enable cheaper and longer-range electric vehicles that strike the right balance between feasibility of commercialization and better performance.
Still, the challenges facing an energy-dense, cost-effective lithium-sulfur battery are formidable. Making it will probably require replacing all of the major components of the lithium-ion battery. Scientists are struggling to prevent the sulfur in the positive electrode, or cathode, from dissolving. And making a required change to the negative electrode or anode—from graphite in lithium-ion batteries to solid lithium metal—has resulted in the electrode developing weird growths (“dendrites”) that can short circuit the battery. Furthermore, researchers are looking for solid materials with which to replace the liquid electrolyte that shuttles ions between the anode and cathode. Still, if they succeed, batteries could store more energy, discharge power more rapidly, and pose fewer safety risks (read: no more fires on planes).
2. R&D is needed not only to invent new materials, but also to scale up manufacturing processes
Argonne’s Materials Engineering Research Facility (MERF) focuses on an often-overlooked aspect of battery innovation—figuring out how to manufacture new materials at commercial scale. Researchers explained to me their rigorous process flow, which includes vetting new materials to see if they can in theory be scaled up, and making larger and larger quantities of the materials. They might modify the chemical composition to synthesize a material invented in the lab to make it easier to manufacture, but if it fails to deliver the same performance on a large scale, researchers must go back to the drawing board. Importantly, researchers use industrial-scale equipment, which differs substantially from lab tools used to make very small quantities of new materials (figure 2).
The logic behind MERF is sound and badly needed. Still, Argonne has a long way to go. So far, MERF has only focused on new materials for lithium-ion batteries, whereas brand new battery technologies are arguably even more in need of such a facility to make up for the industry’s apathy toward emerging battery architectures. And even within the lithium-ion battery space, MERF has struggled to produce a game-changing new material at a scale that battery manufacturers are willing to license. Still, MERF has had some limited examples of successful licensing arrangements. And I’m positive that it will take many more MERF-like facilities—scaling up the manufacturing of batteries as well as other clean energy technologies—to realize the potential of national laboratory discoveries.

3. International R&D cooperation is about much more than technology development
Recently, the U.S.-China Clean Energy Research Center (CERC) was renewed for another five-year collaboration, and Argonne was chosen to lead the Clean Vehicle Consortium (CVC) from the U.S. side. I was struck by the CVC’s range of goals—over and above developing new technologies in the lab. The collaboration could be a vehicle (pun intended) for U.S. firms to bring advanced battery and vehicle technologies to the Chinese market. And it could offer a venue for senior U.S. and Chinese officials to meet with each other and discuss not only CERC’s progress but also completely unrelated diplomatic priorities. Amazingly, clean energy technology (along with climate change—though friction remains on aspects of climate negotiations) has emerged as the brightest spot in U.S.-China relations, uplifting a relationship that has been otherwise marred by tensions around the South China Sea, cybersecurity, and more.
The downside may be that genuine technology development could become a casualty of the pressure on CERC to deliver on broader diplomatic goals. Joint patenting of technology with China has been rare, as U.S. companies in particular remain wary of China’s intellectual property protections. Still, I got the sense that CVC’s collaboration on driverless, networked vehicles is an effort where both the U.S. and Chinese sides are committed to jointly developing new technologies in a largely unexplored area.
4. National laboratories and entrepreneurs could be natural partners
Building on a model pioneered at Lawrence Berkeley National Laboratory called “Cyclotron Road,” Argonne is preparing to kick off its own incubator for clean energy technology entrepreneurs to take advantage of the extensive resources at the Lab. Dubbed “Chain Reaction Innovations” (CRI), the program is about to announce its inaugural class of entrepreneurs, pairing them with veteran Argonne researchers to develop their technologies on campus. I’ve argued before that this model is crucial for entrepreneurs to get their start without venture capitalists hanging a Sword of Damocles over their heads by infusing their start-ups with capital but expecting speedy and lucrative payouts.
Now, national labs around the country are looking into hosting their own classes of entrepreneurs, and it will likely take many such programs to boost clean energy technology in the wake of the start-up bubble collapse. But in this area as in others, I came away from my visit reassured that Argonne is looking ahead to play a vital role in bringing clean energy breakthroughs to market.
I am very grateful to my hosts at Argonne National Laboratory, including Peter Littlewood, Greg Morin, Dave Hooper, Julie Wulf-Knoerzer, Don Hillebrand, Kris Pupek, Norm Peterson, Suresh Sunderajan, Andreas Roelofs, Kevin Gallagher, Brad Ullrick, and Devin Hodge.
More on:
 Online Store
Online Store